CDSCO Urges States to Withdraw NOC for Opioid Drug Combination
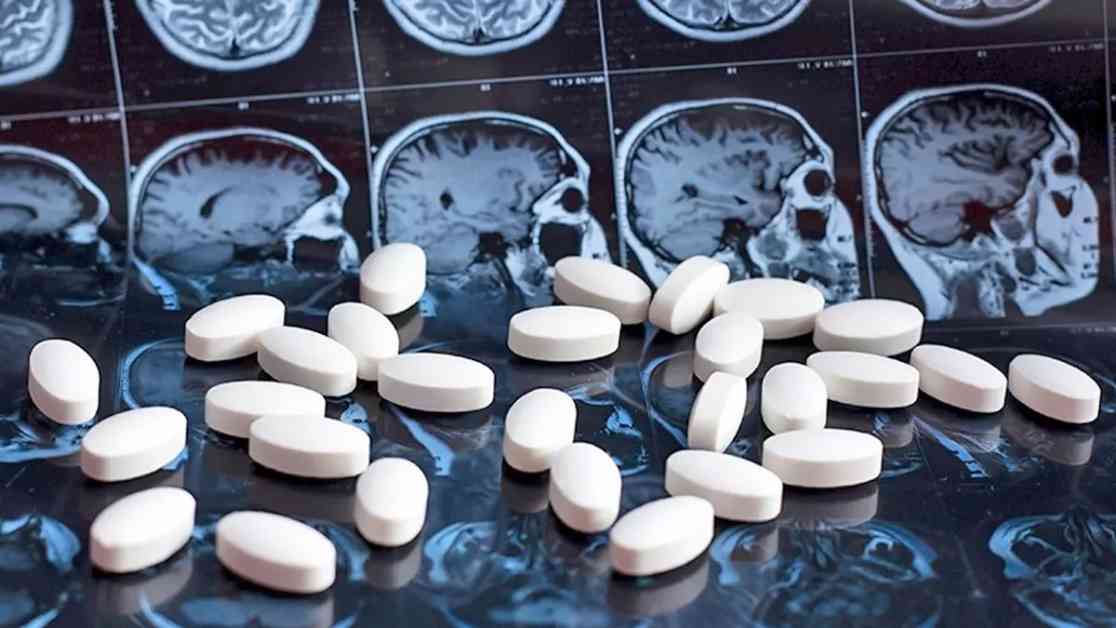
In a recent development, the Central Drugs Standard Control Organisation (CDSCO) has issued a directive to State and Union Territories’ drug control authorities, urging them to revoke the NOC (no objection certificate) for the export and manufacture of a combination of opioid painkiller Tapentadol and muscle-relaxant Carisoprodol. This decision comes in light of a report indicating the abuse of these drugs in West African countries.
Immediate Action Required
The CDSCO’s communication also includes a request to withdraw the NOCs for the export and manufacture of all combinations involving these two drugs, independently, if they are not approved by the importing country. This directive is set to take effect immediately, highlighting the urgency and gravity of the situation at hand.
Concerns Over Abuse Potential
According to a BBC report cited by the CDSCO, the combination of these drugs presents a “significant potential for abuse” when exported to West African nations from India. Specifically, the report points to Mumbai-based Aveo Pharmaceuticals as the source of these opioid pills, which have allegedly contributed to a public health crisis in Ghana, Nigeria, and the Ivory Coast.
Expert Insights and Industry Response
In response to these allegations, Aveo Pharmaceuticals has yet to provide a formal response to inquiries. However, industry insiders have shed light on the situation, emphasizing the potency of the drug combination. They note that such products undergo intense scrutiny and require meticulous documentation due to their high risk of abuse.
This development comes on the heels of previous incidents involving contaminated cough syrup in Gambia and Uzbekistan in 2022, where products from Indian companies were potentially linked to child deaths. In the aftermath of these tragedies, Indian authorities mandated rigorous testing of pharmaceutical products intended for export. Additionally, the top 300 brands in the domestic market were required to implement track and trace codes to enhance regulatory oversight.
Ensuring Public Safety
The CDSCO’s directive underscores the government’s commitment to safeguarding public health and addressing concerns related to the abuse of potent pharmaceuticals. By taking proactive measures to restrict the export and manufacture of opioid drug combinations with abuse potential, authorities aim to prevent further harm and protect vulnerable populations both domestically and internationally.
Looking Ahead
As the pharmaceutical industry continues to navigate challenges related to drug safety and regulatory compliance, stakeholders must prioritize transparency, accountability, and adherence to best practices. By fostering a culture of responsibility and ethical conduct, companies can contribute to a safer, more sustainable healthcare ecosystem that prioritizes patient well-being above all else.